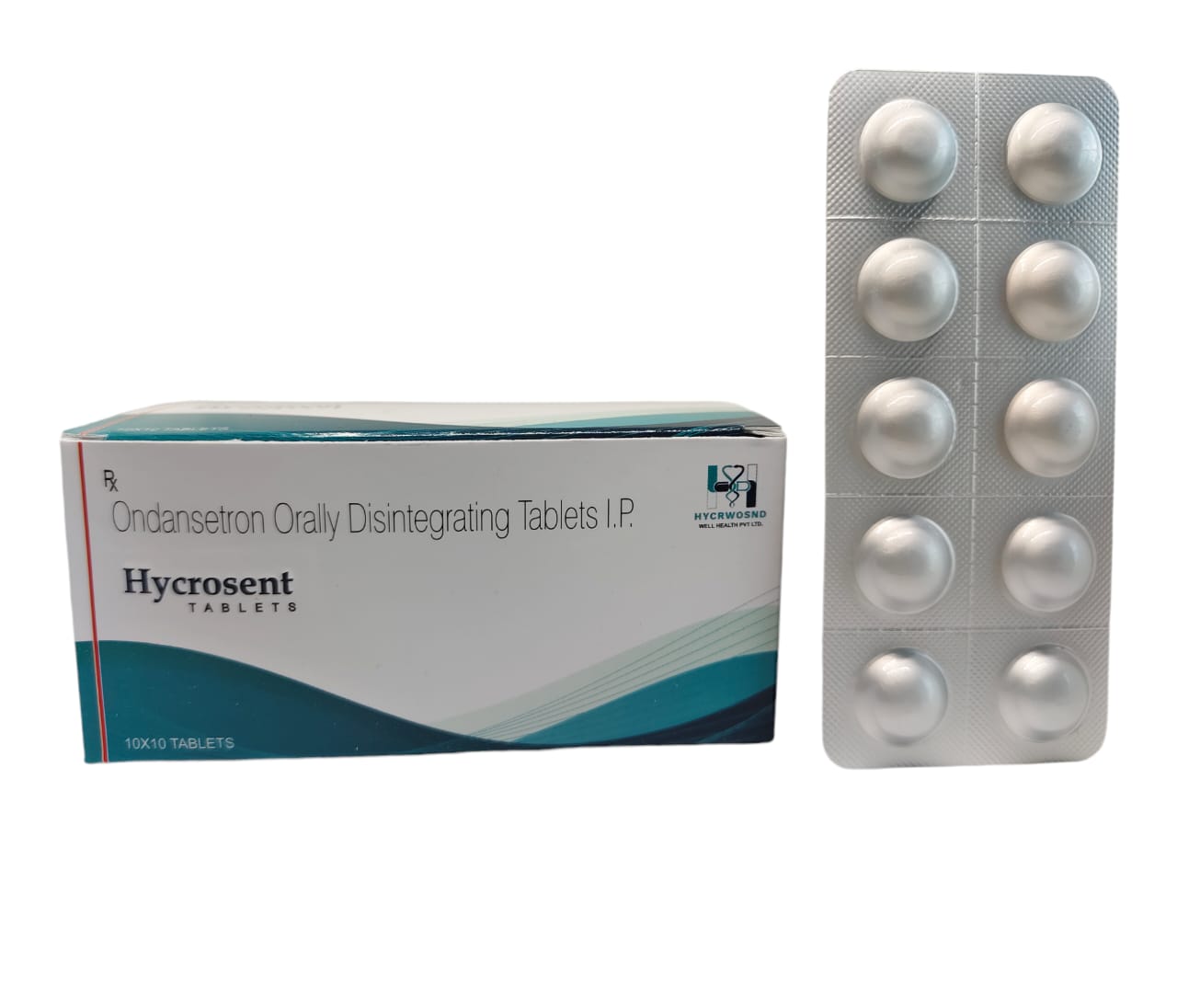
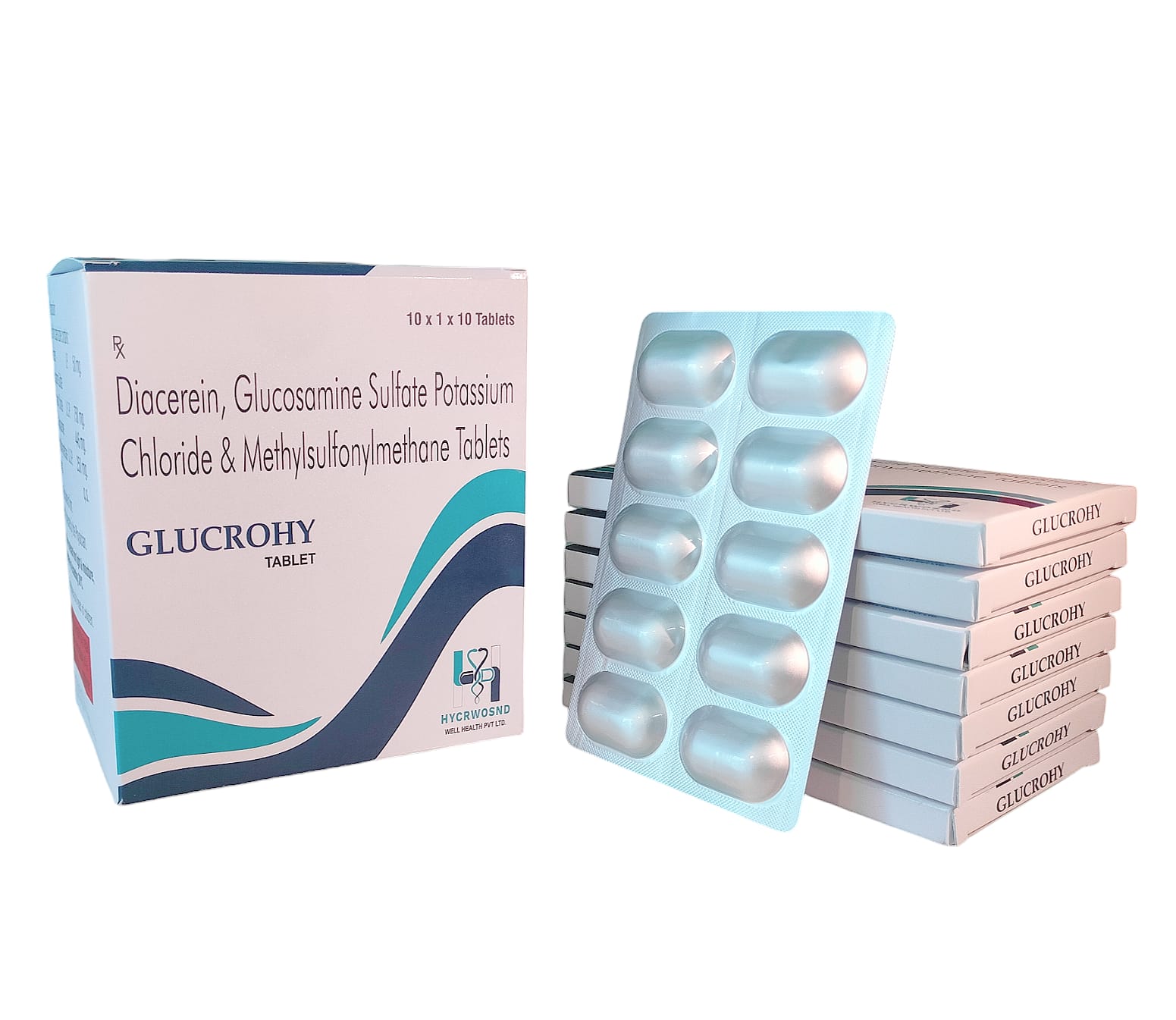
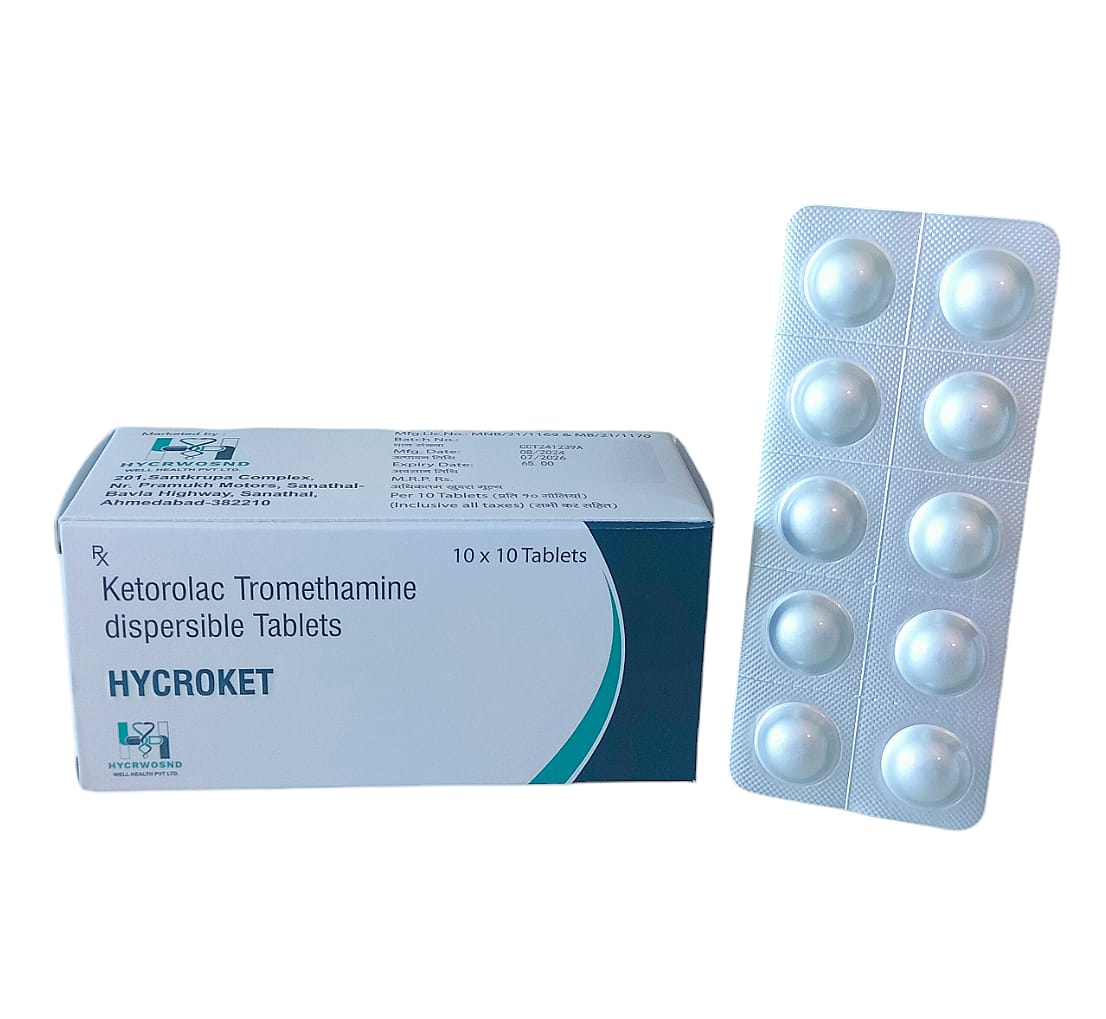
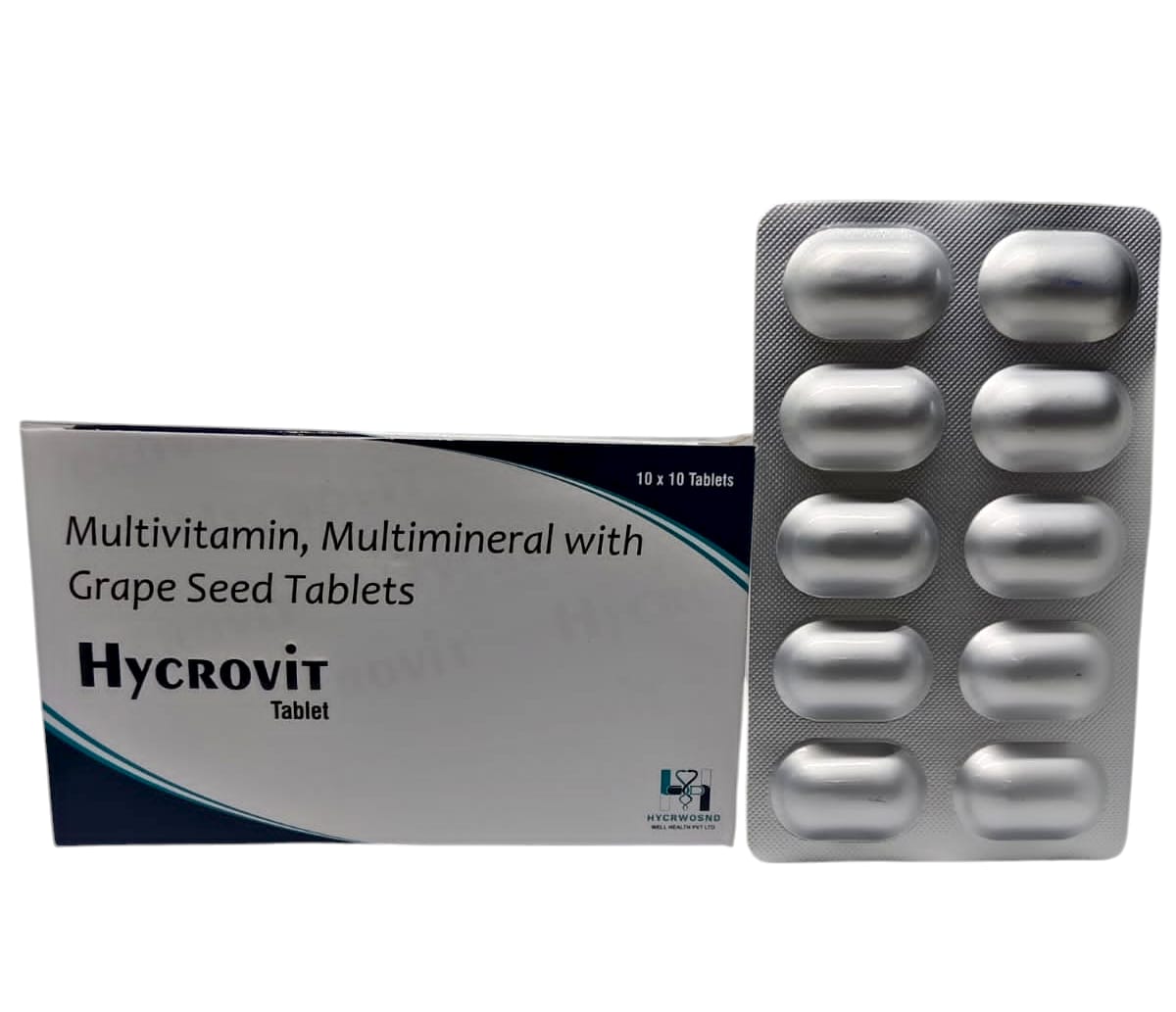
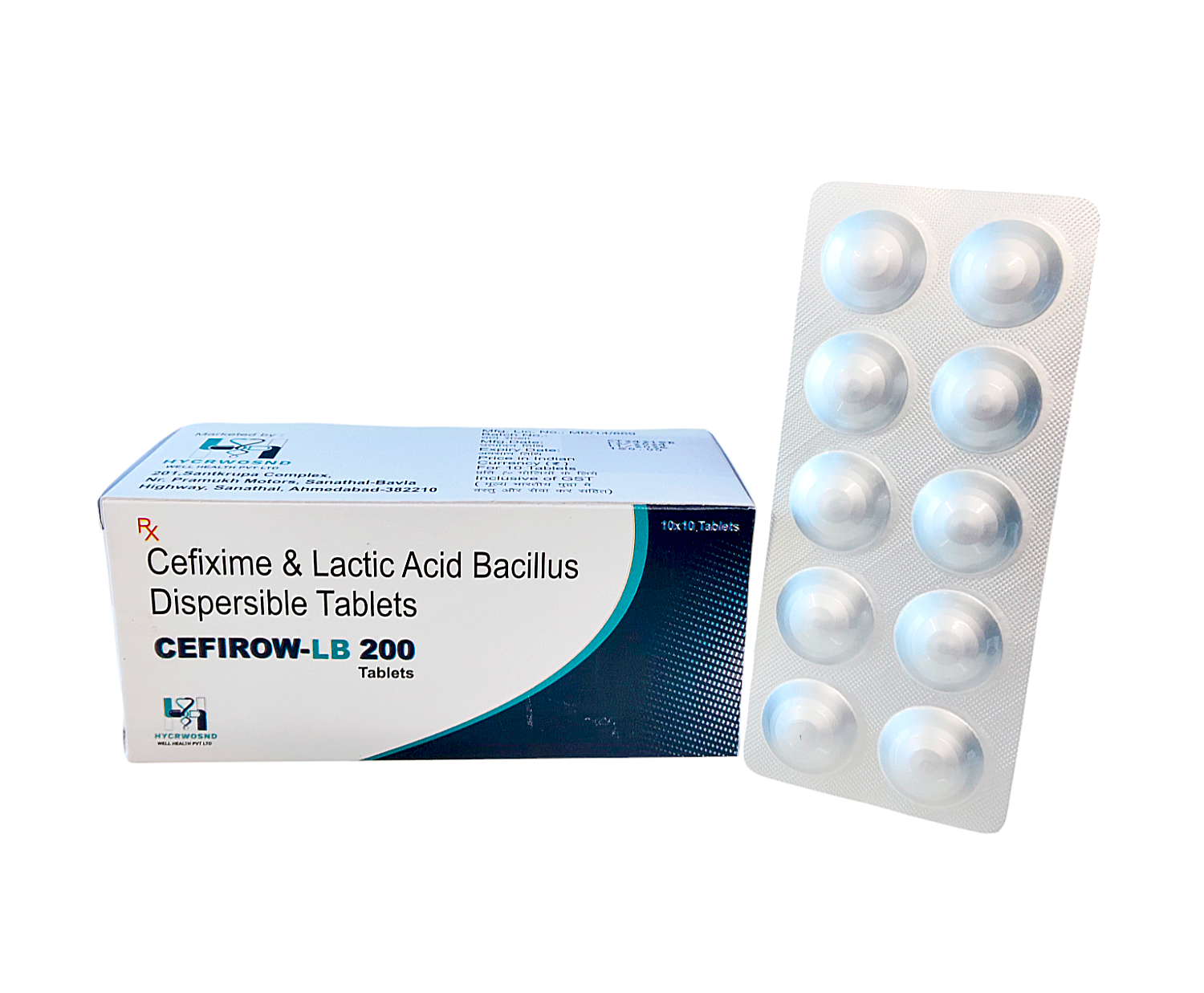
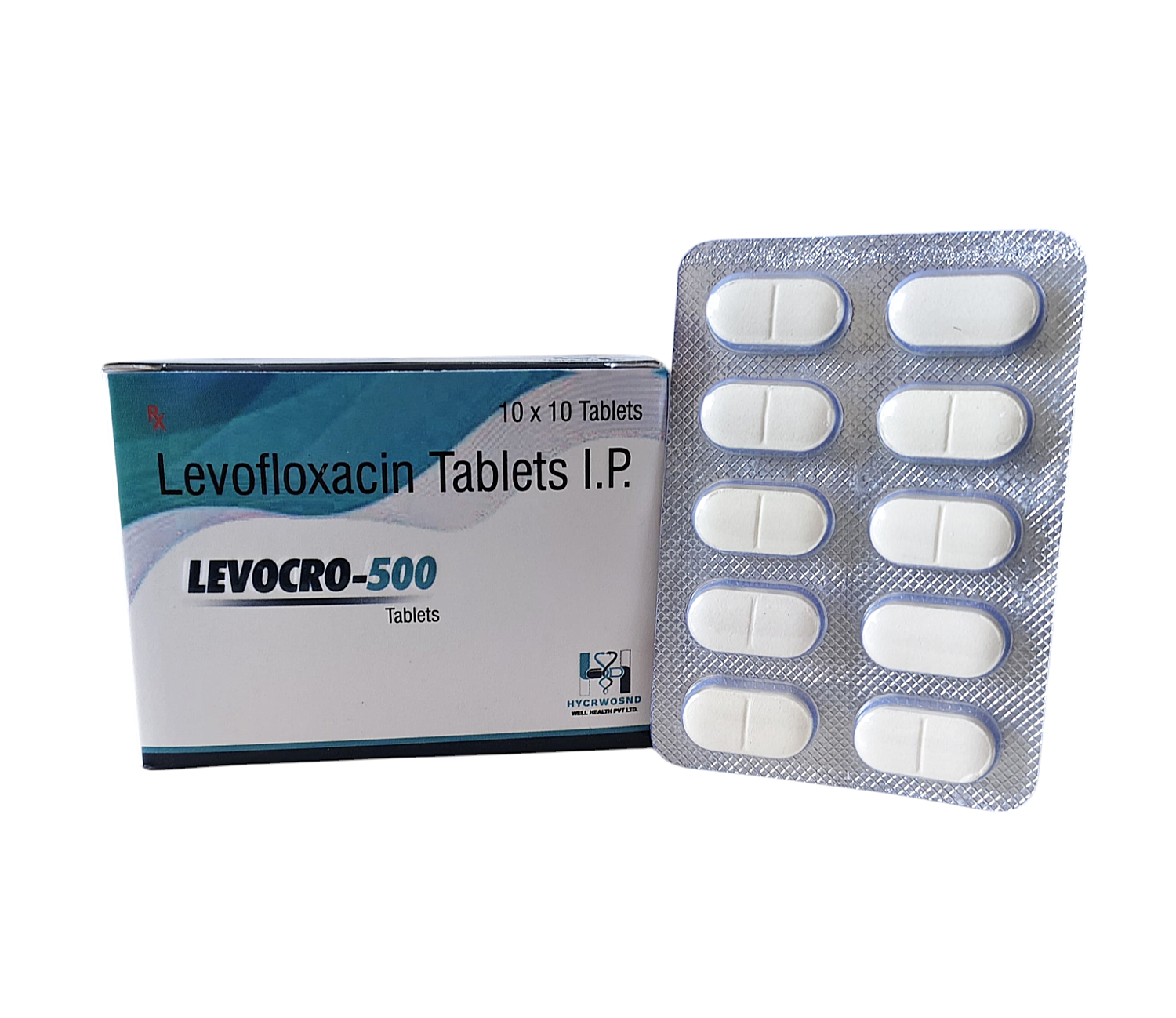
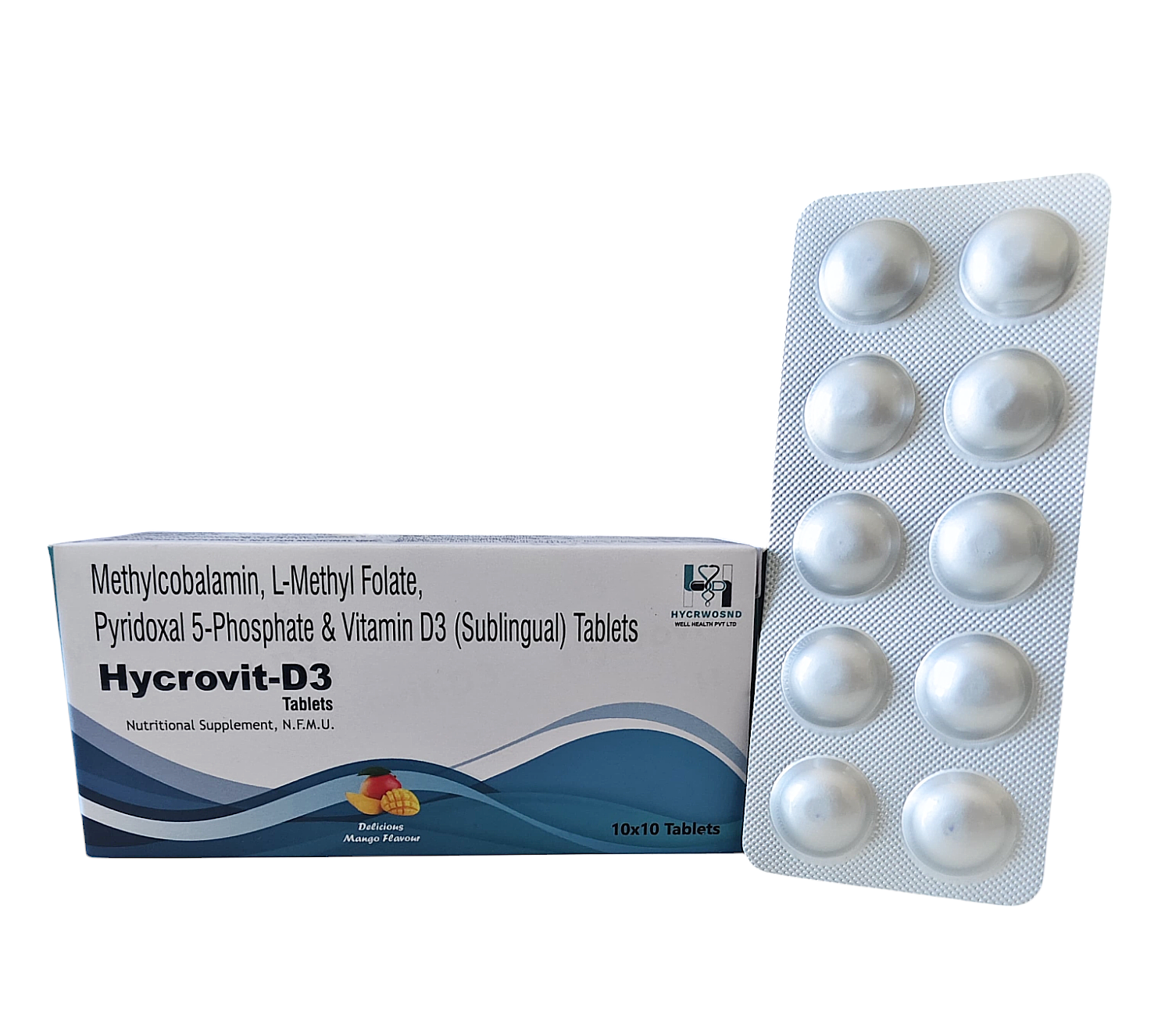
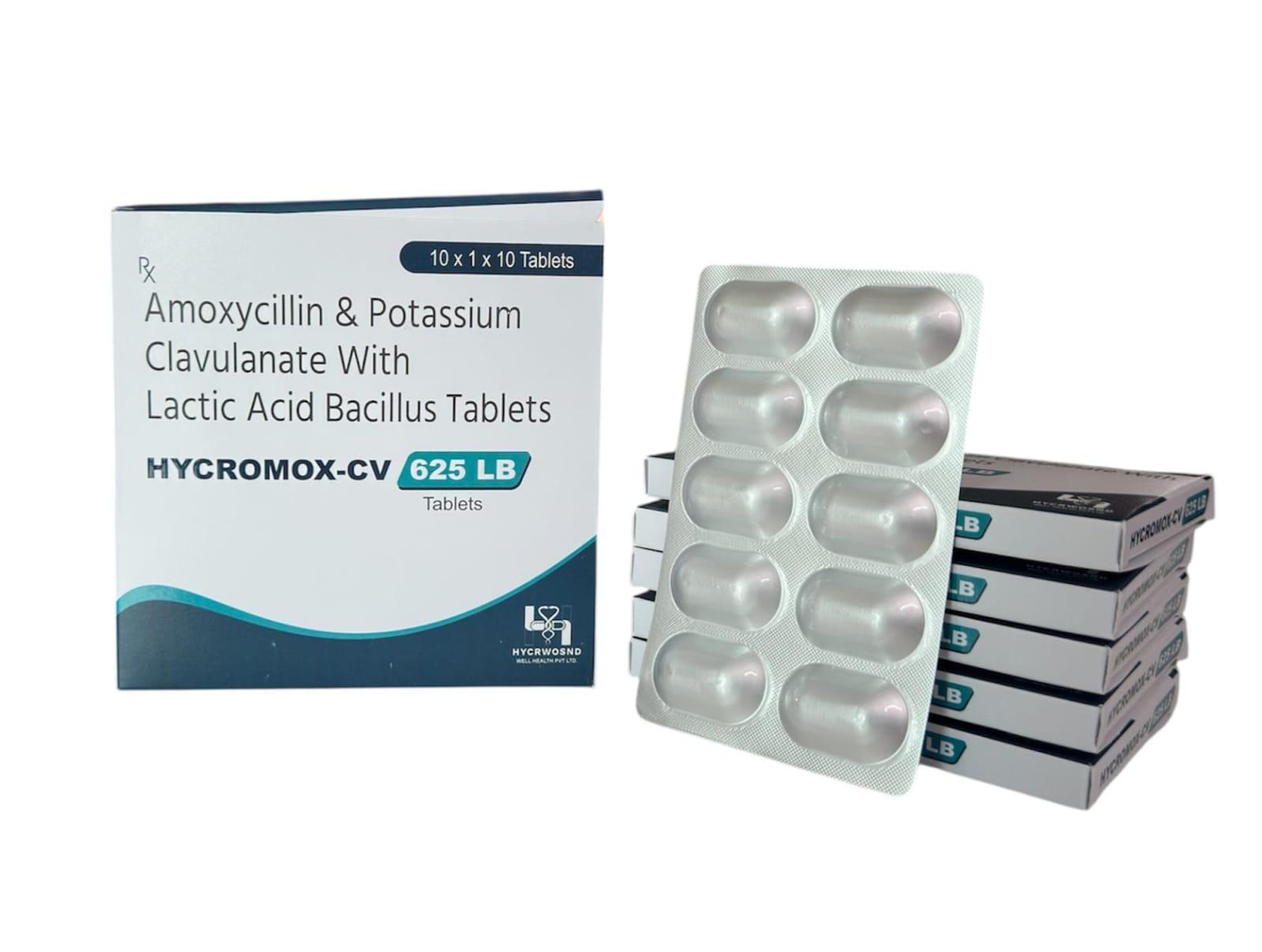
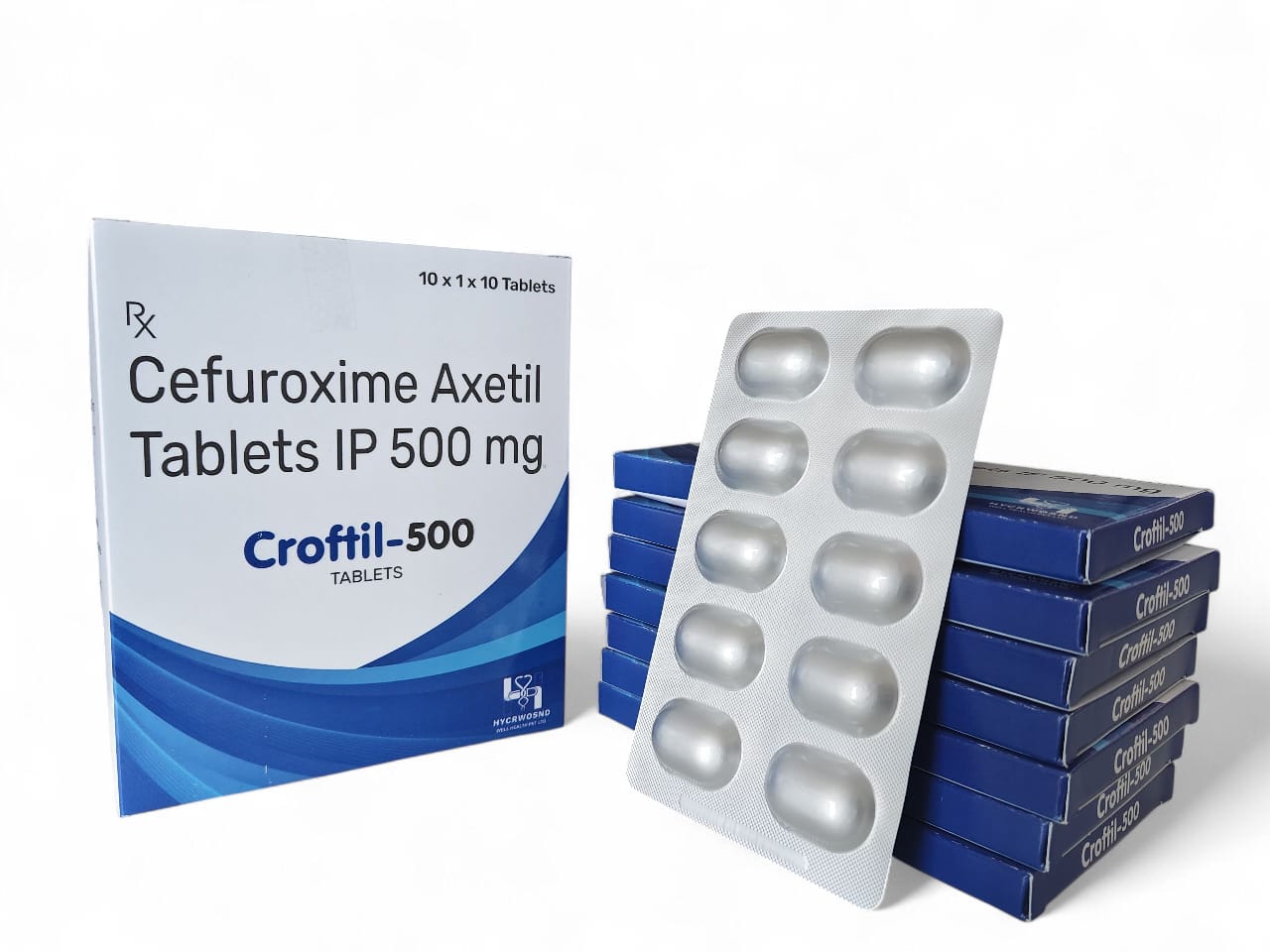
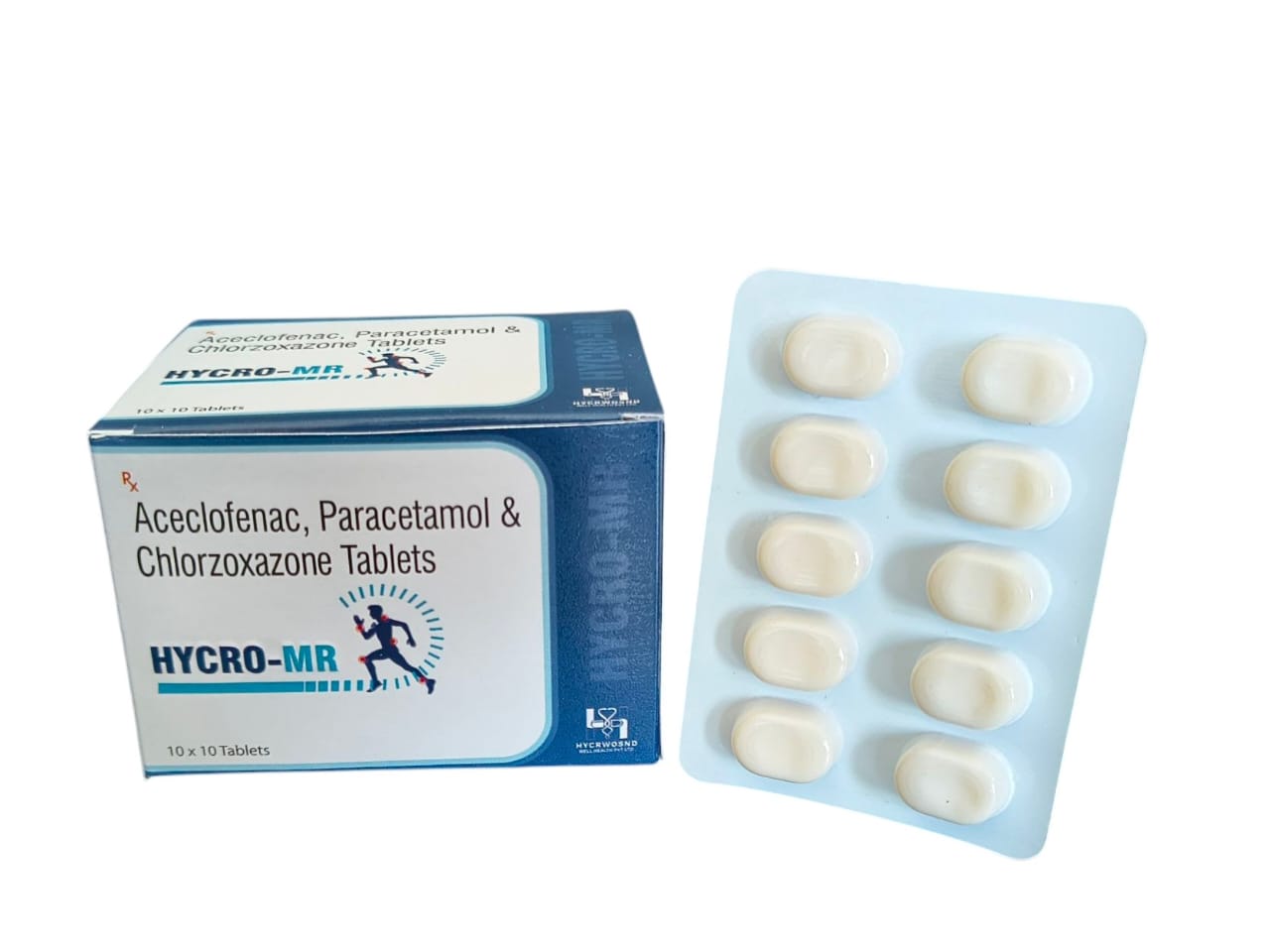
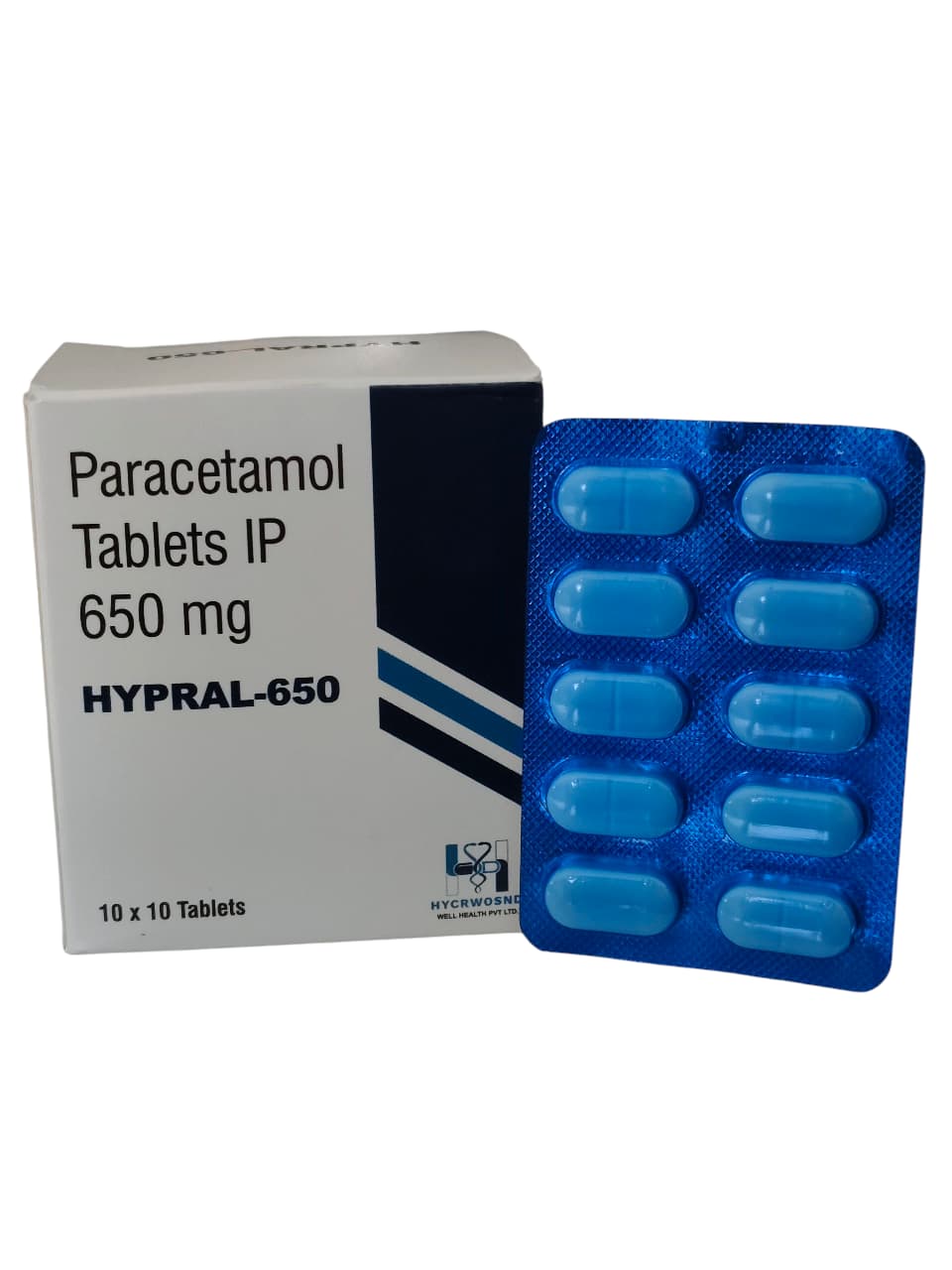
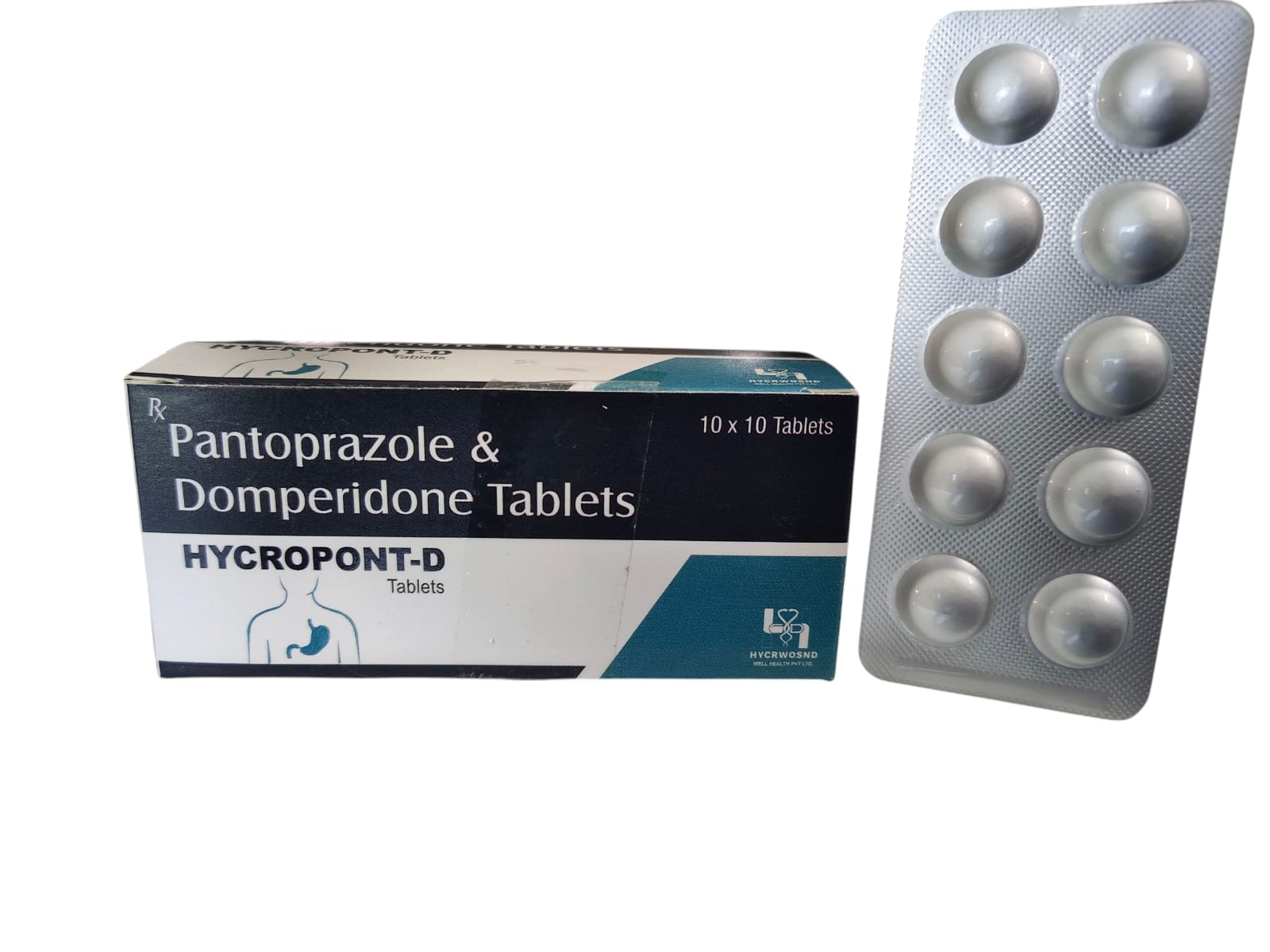
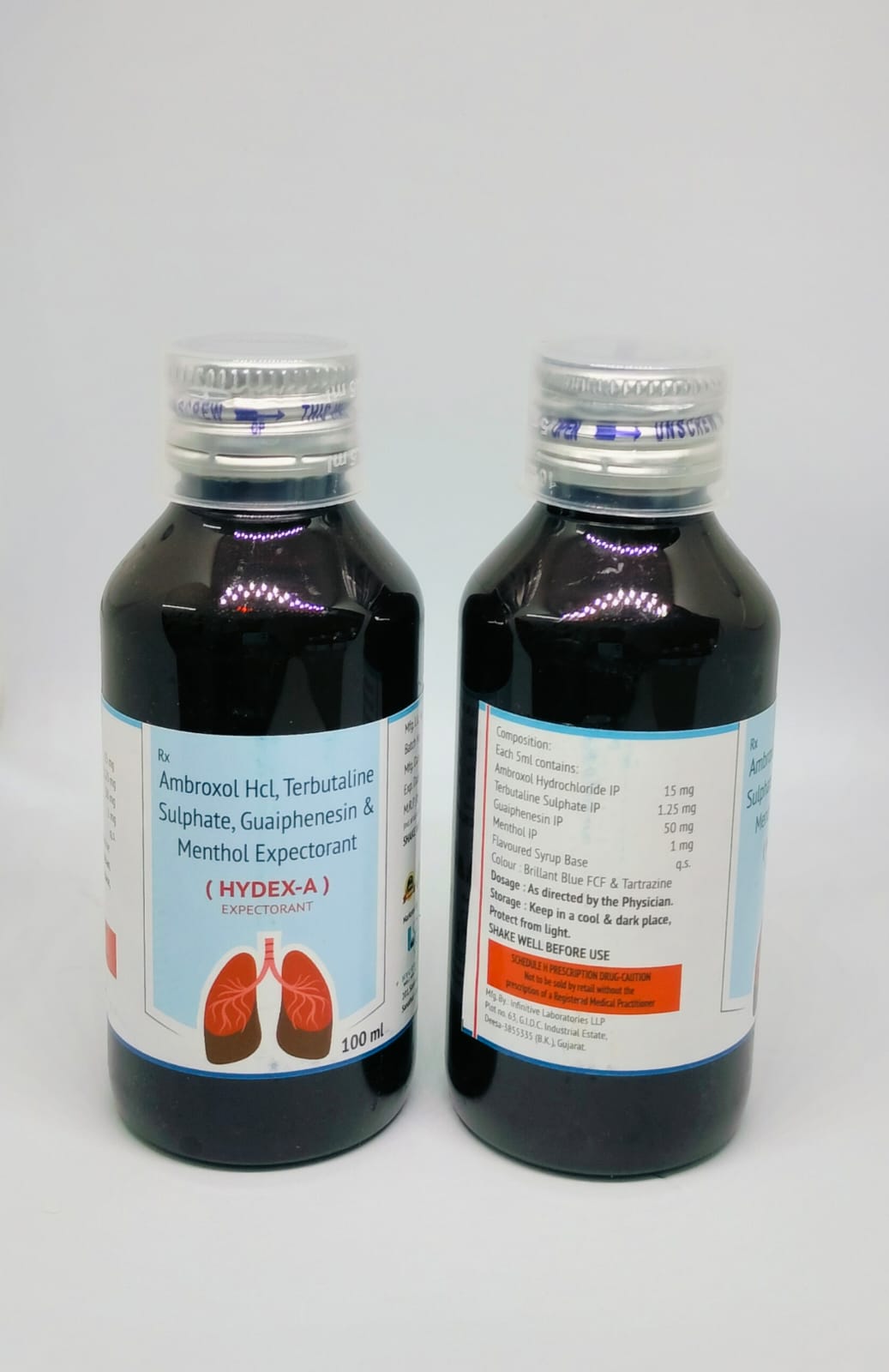
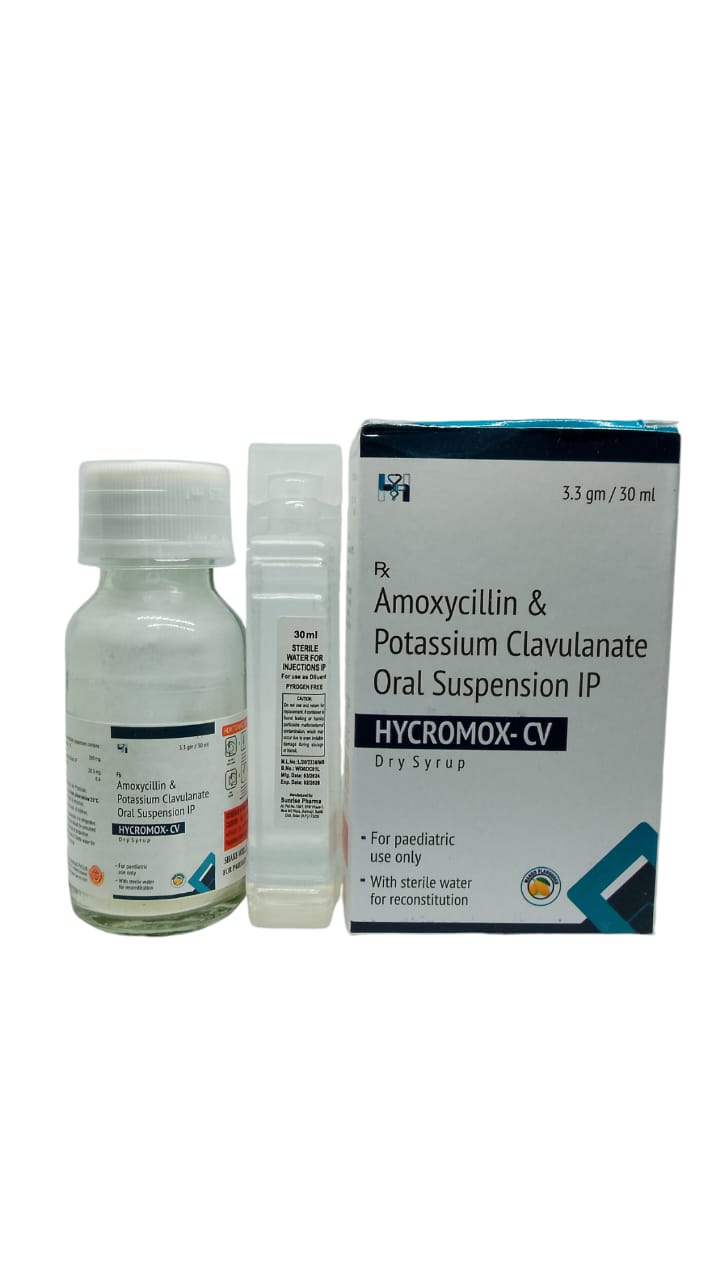
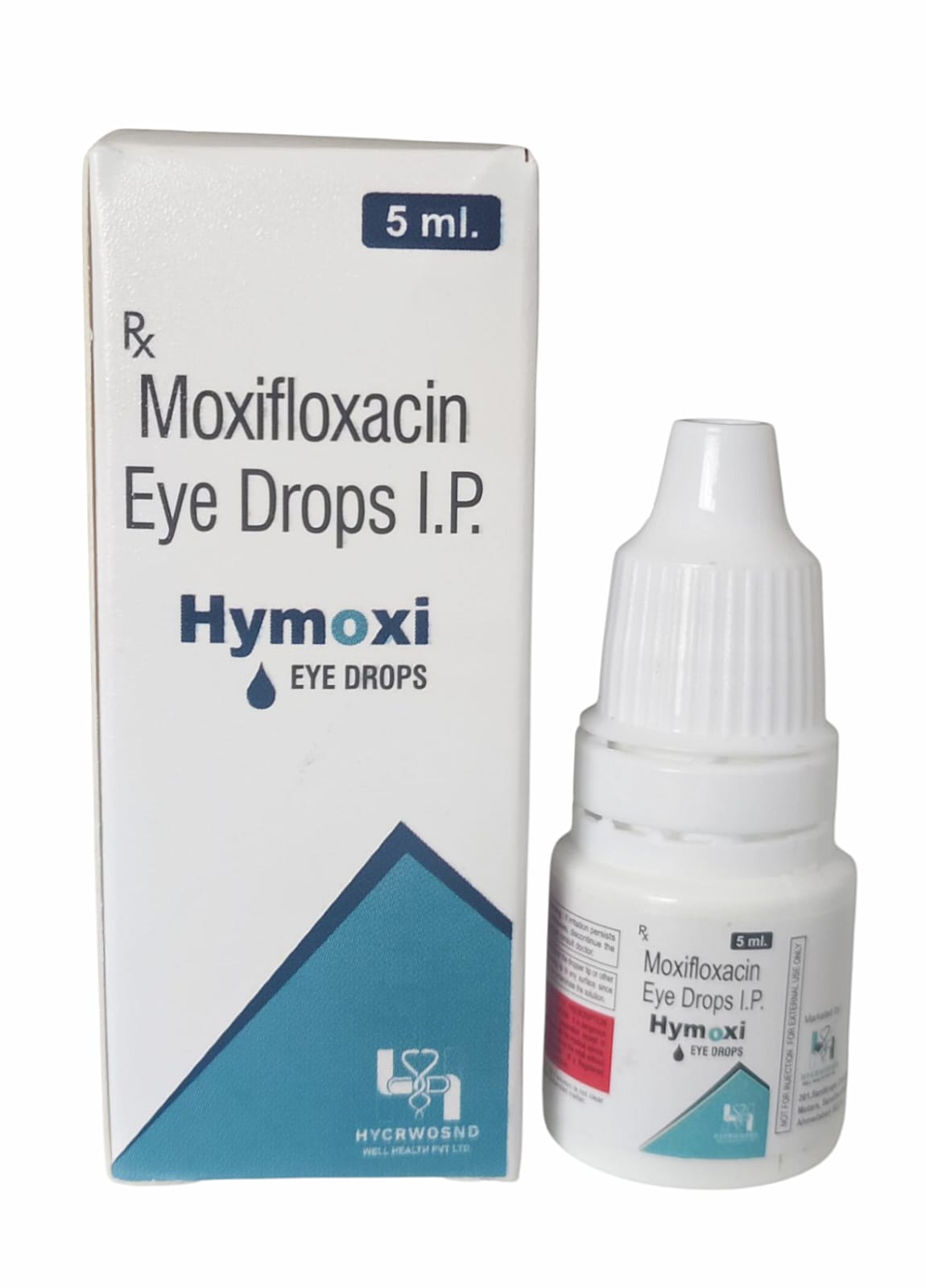
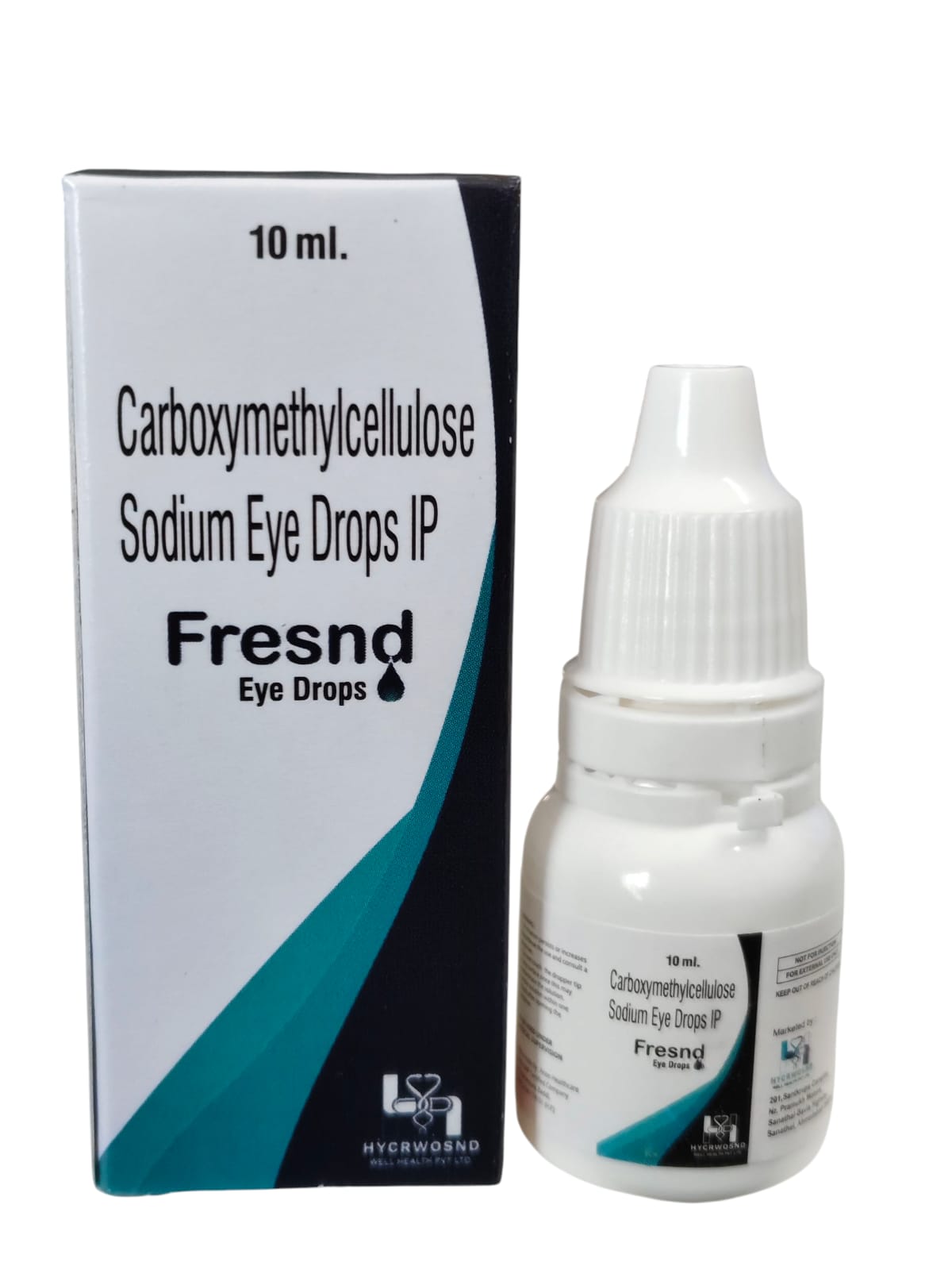
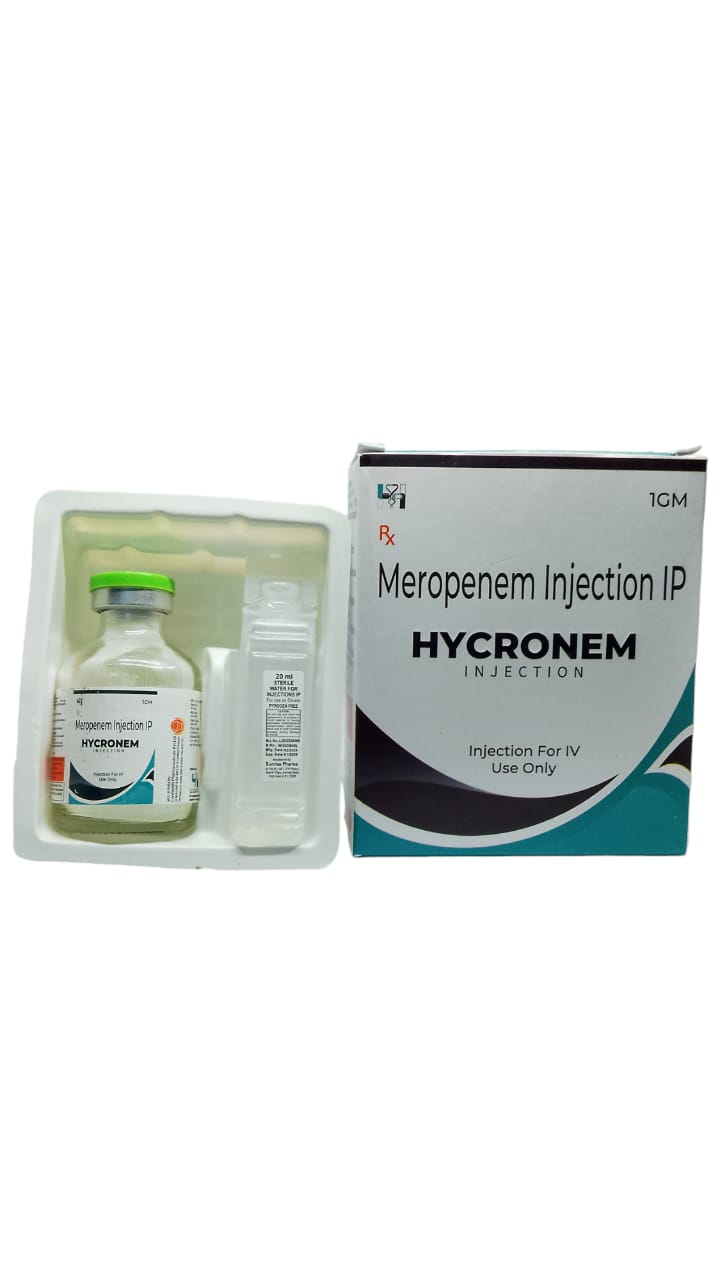
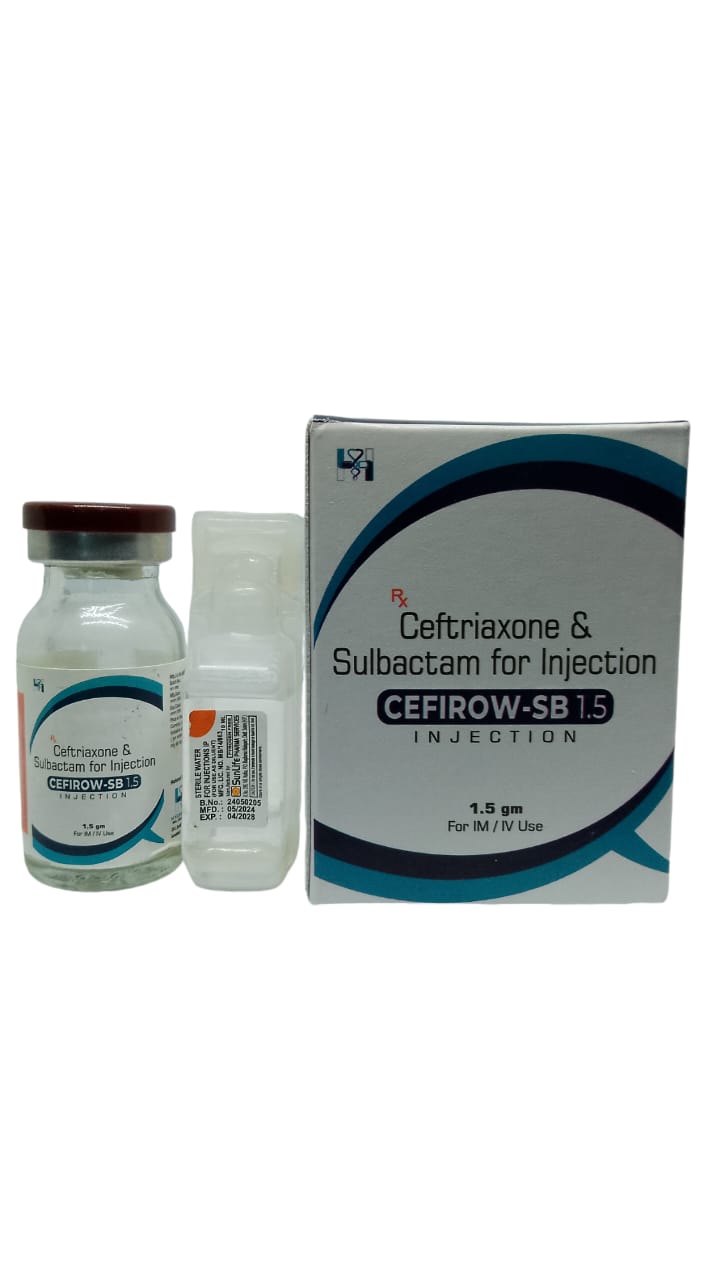
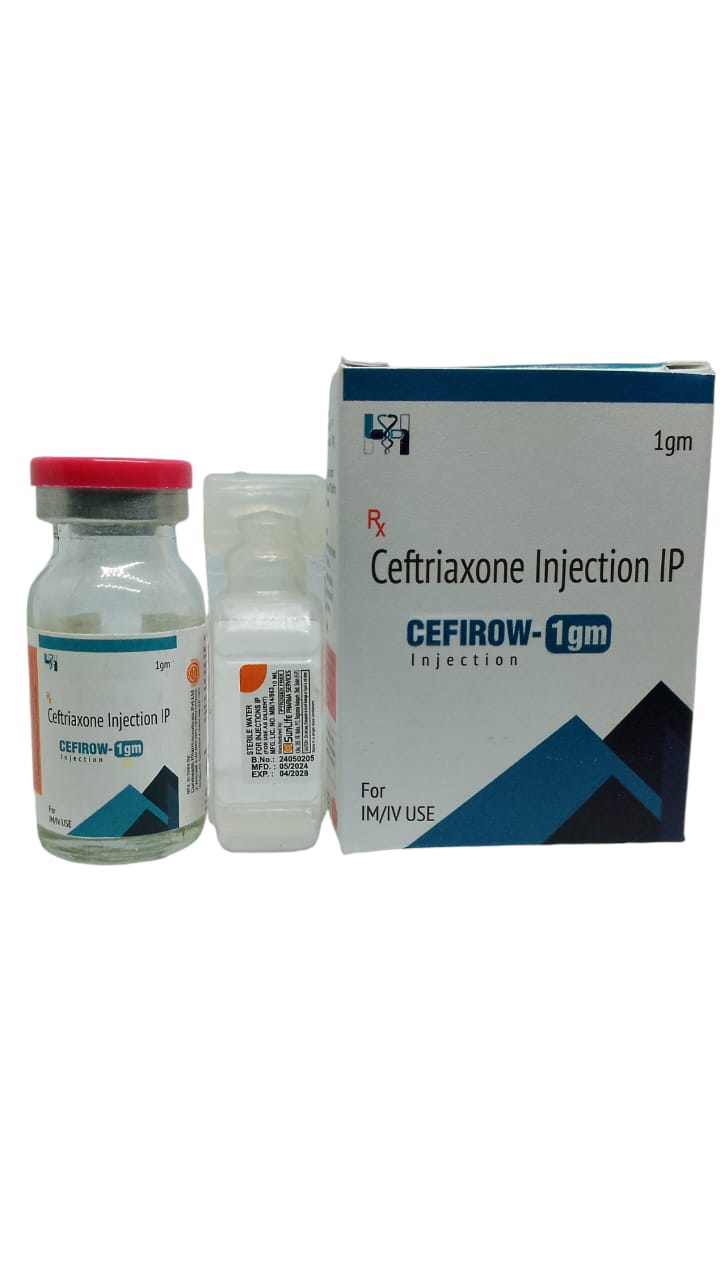
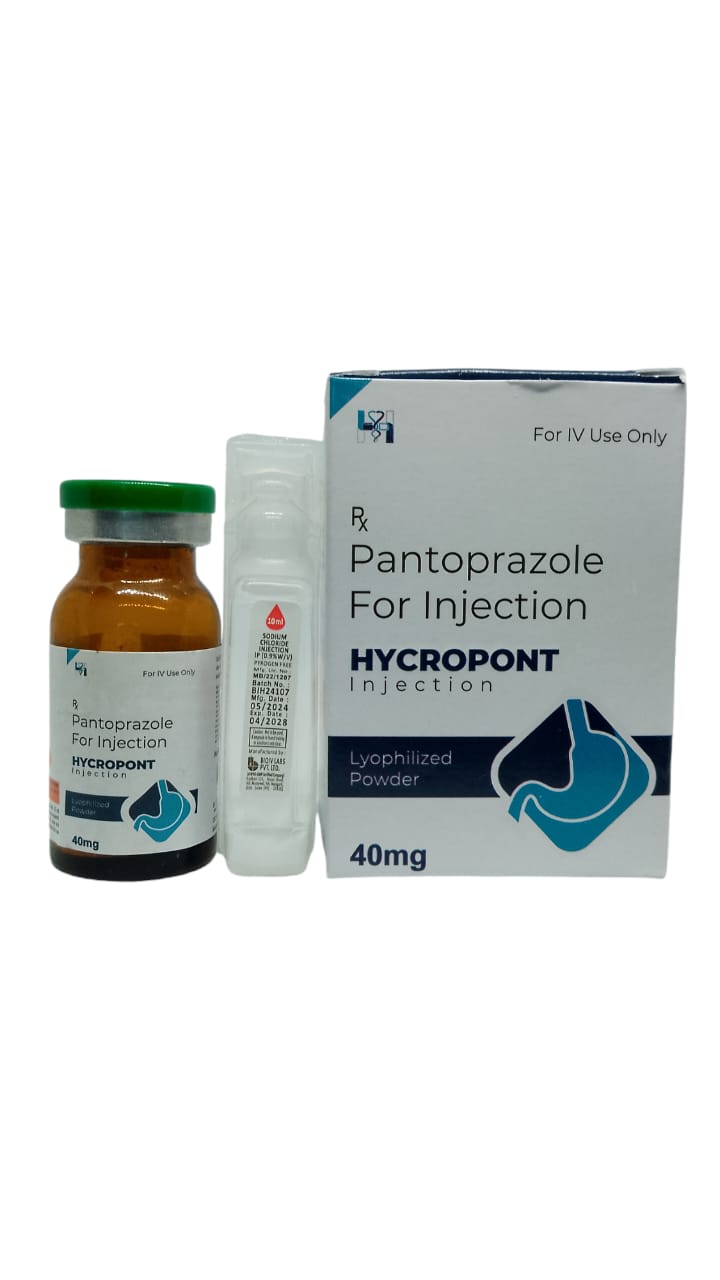
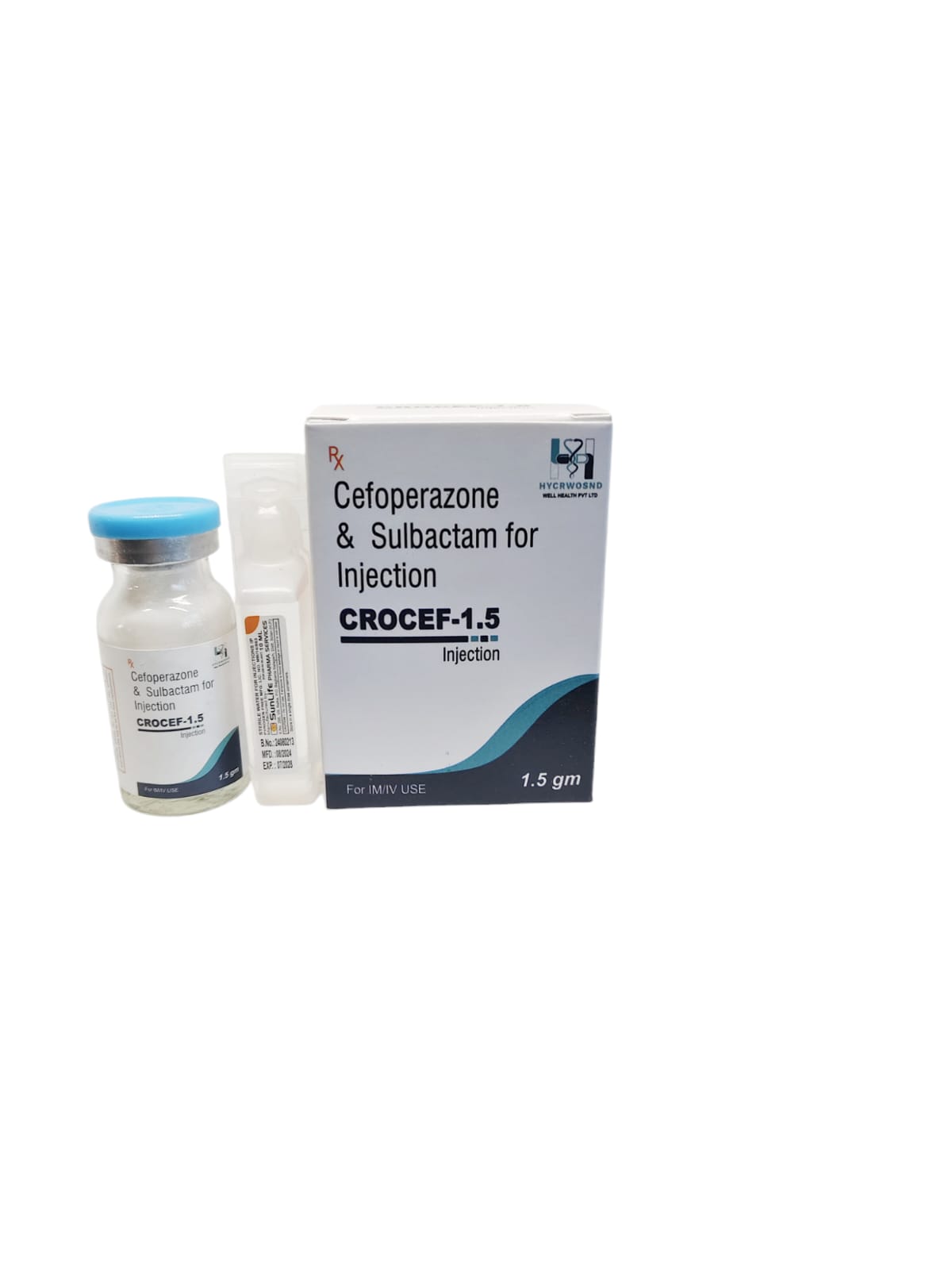
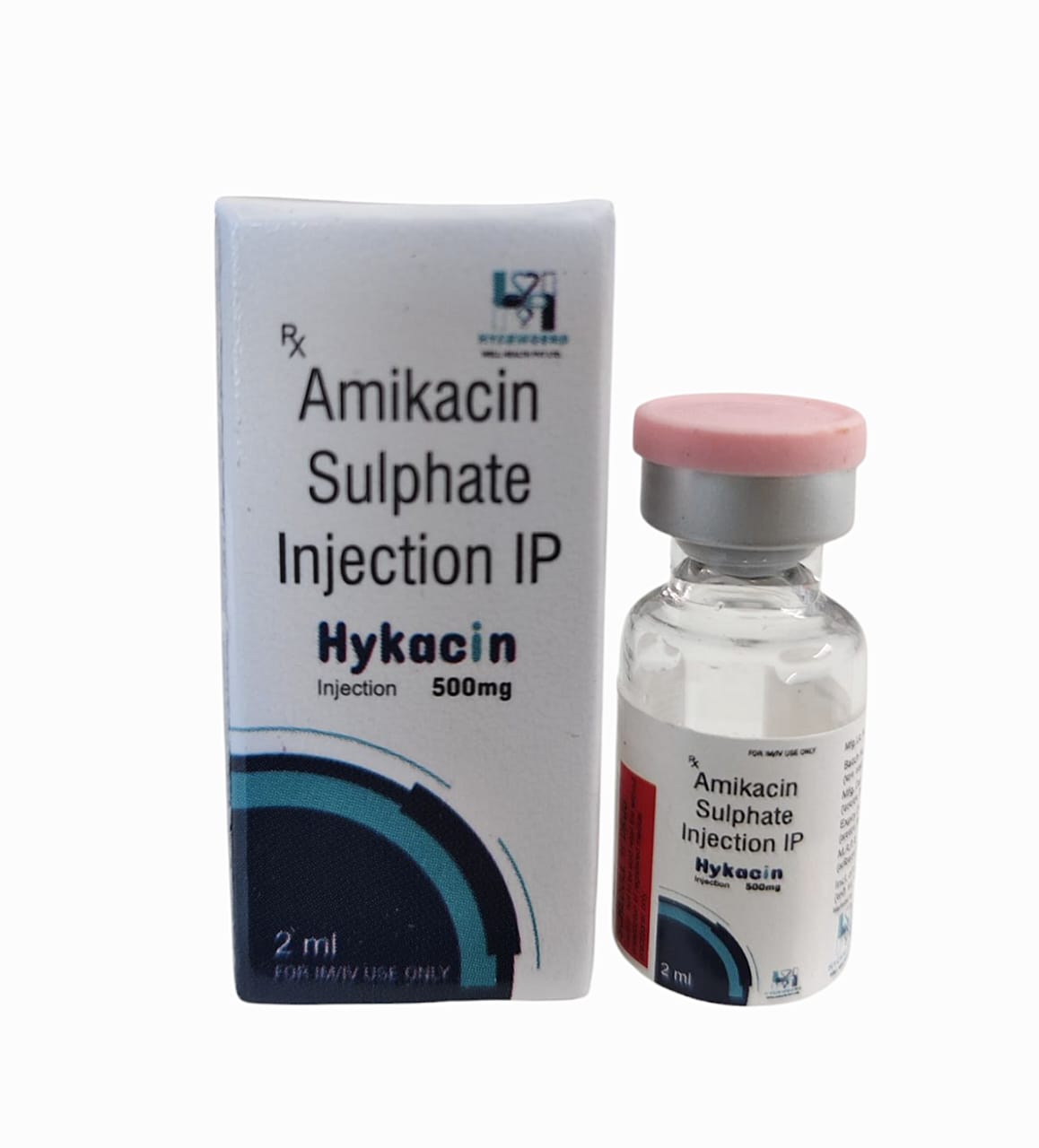
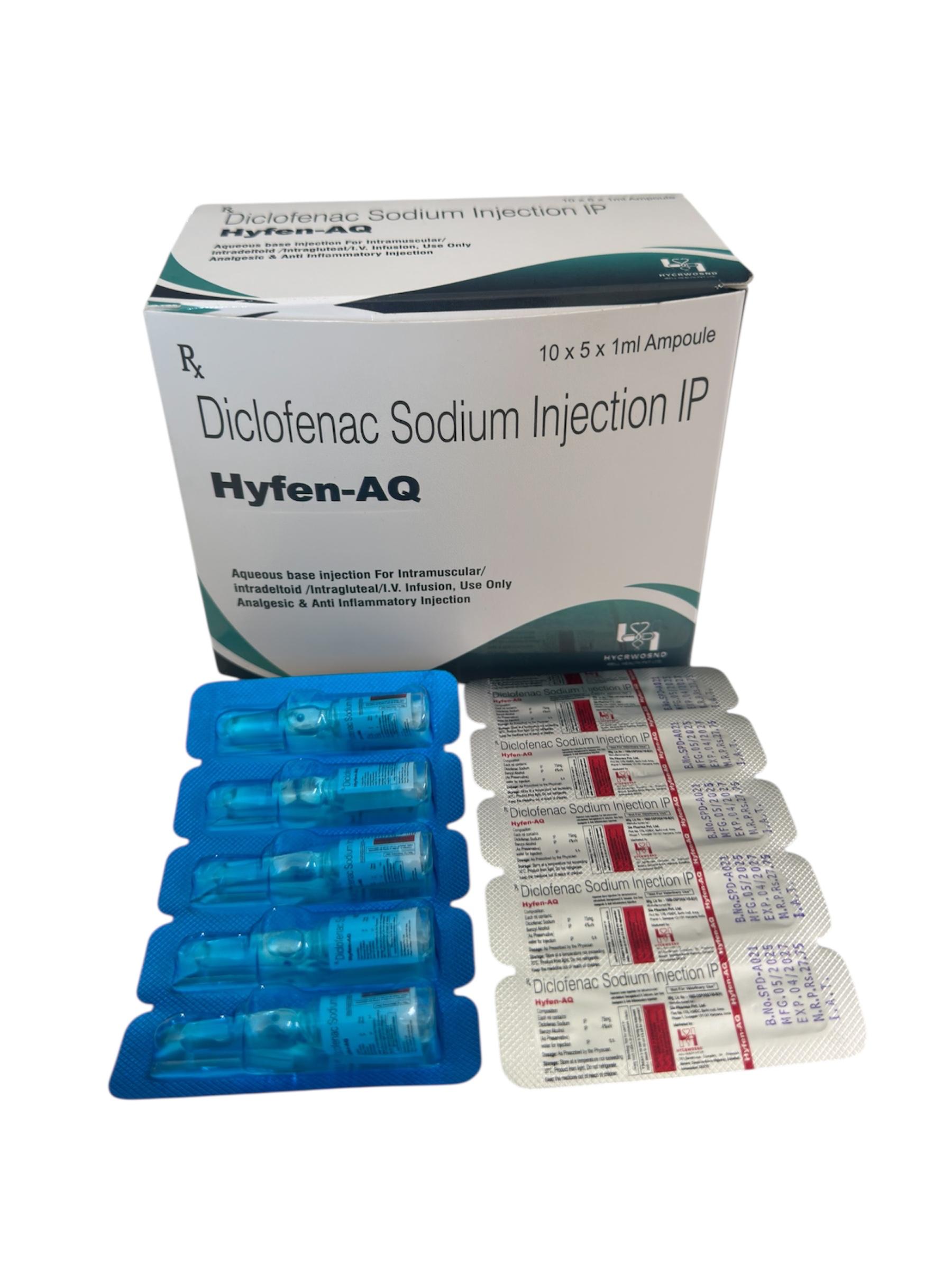
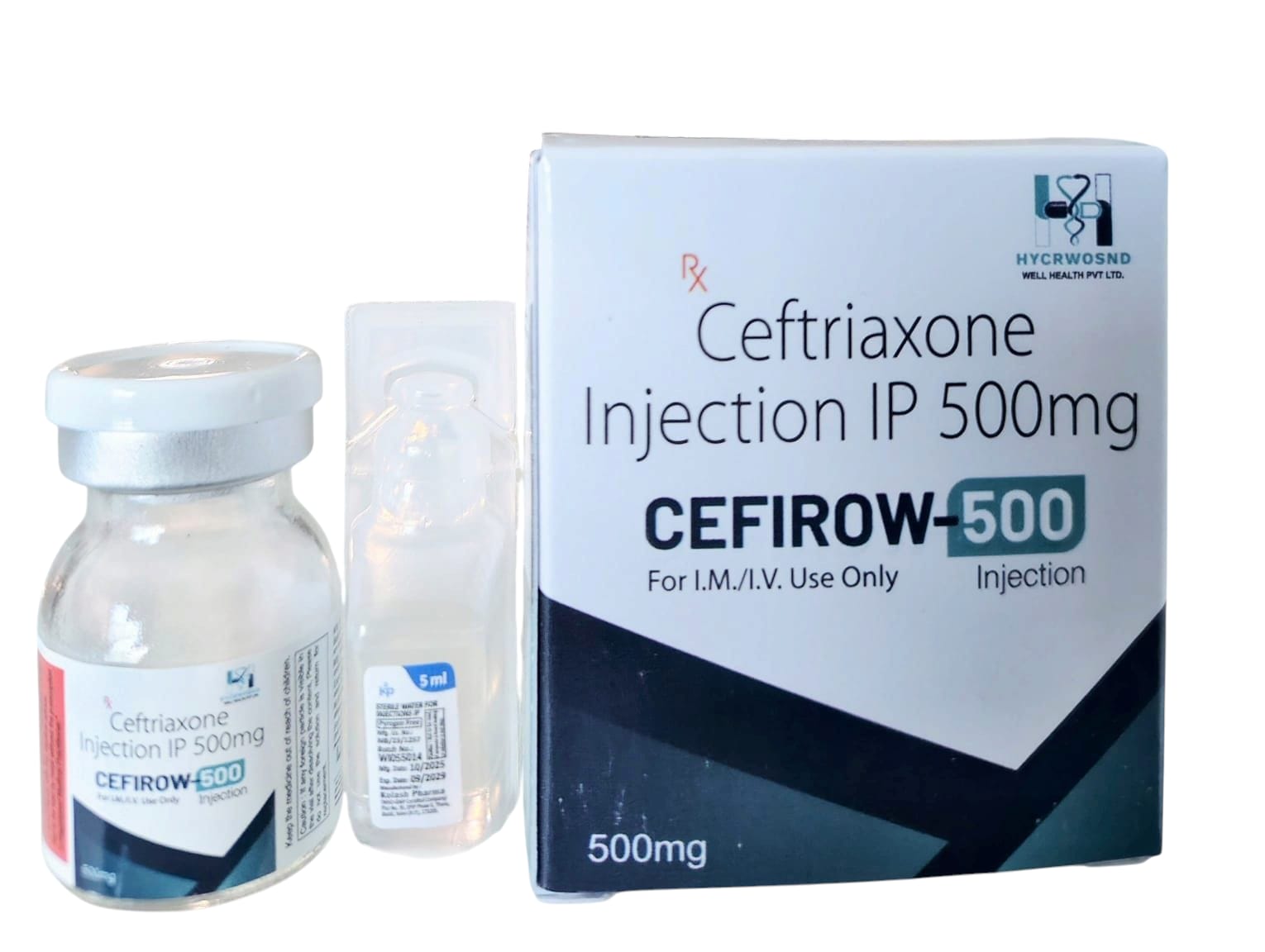
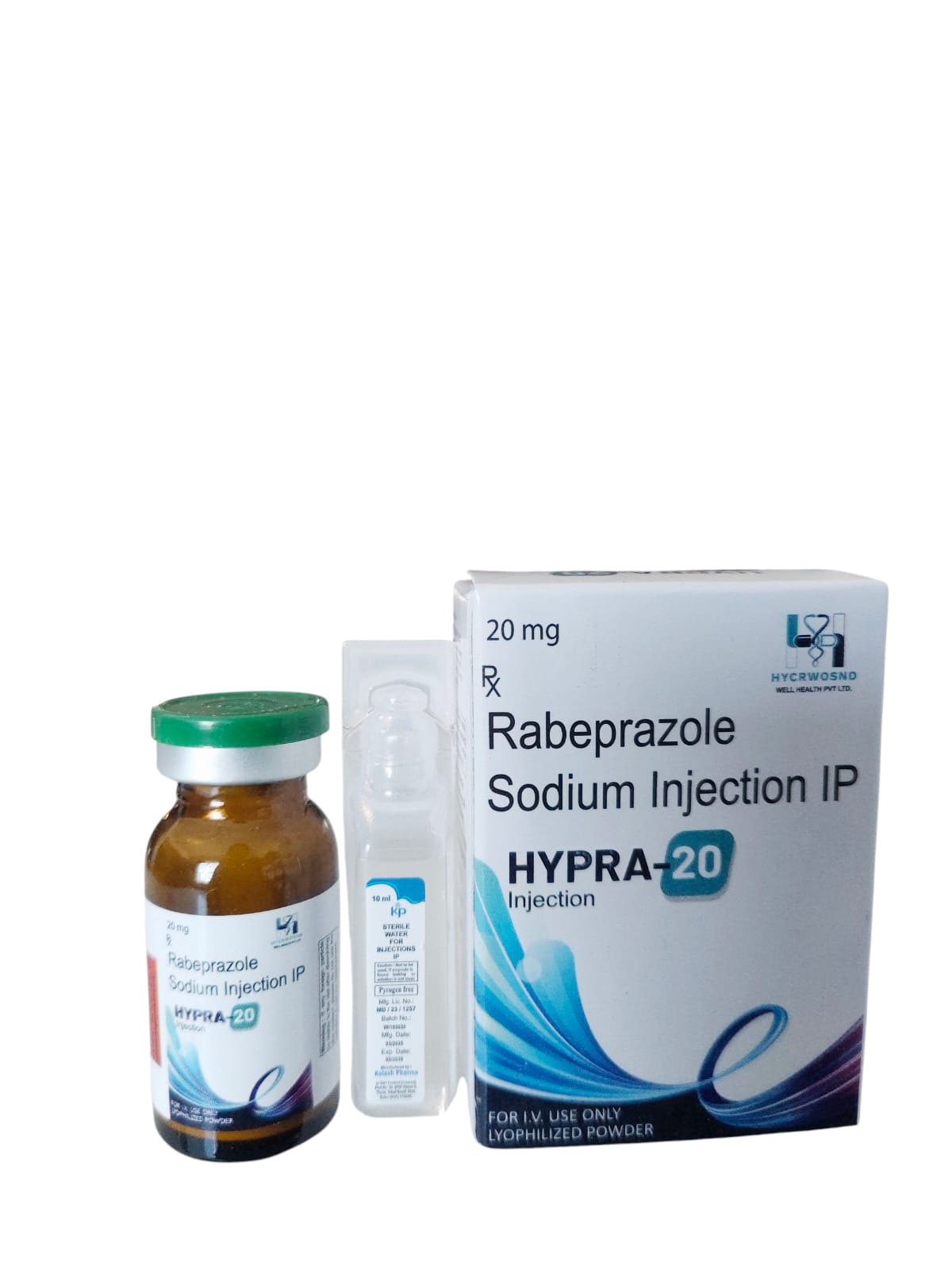
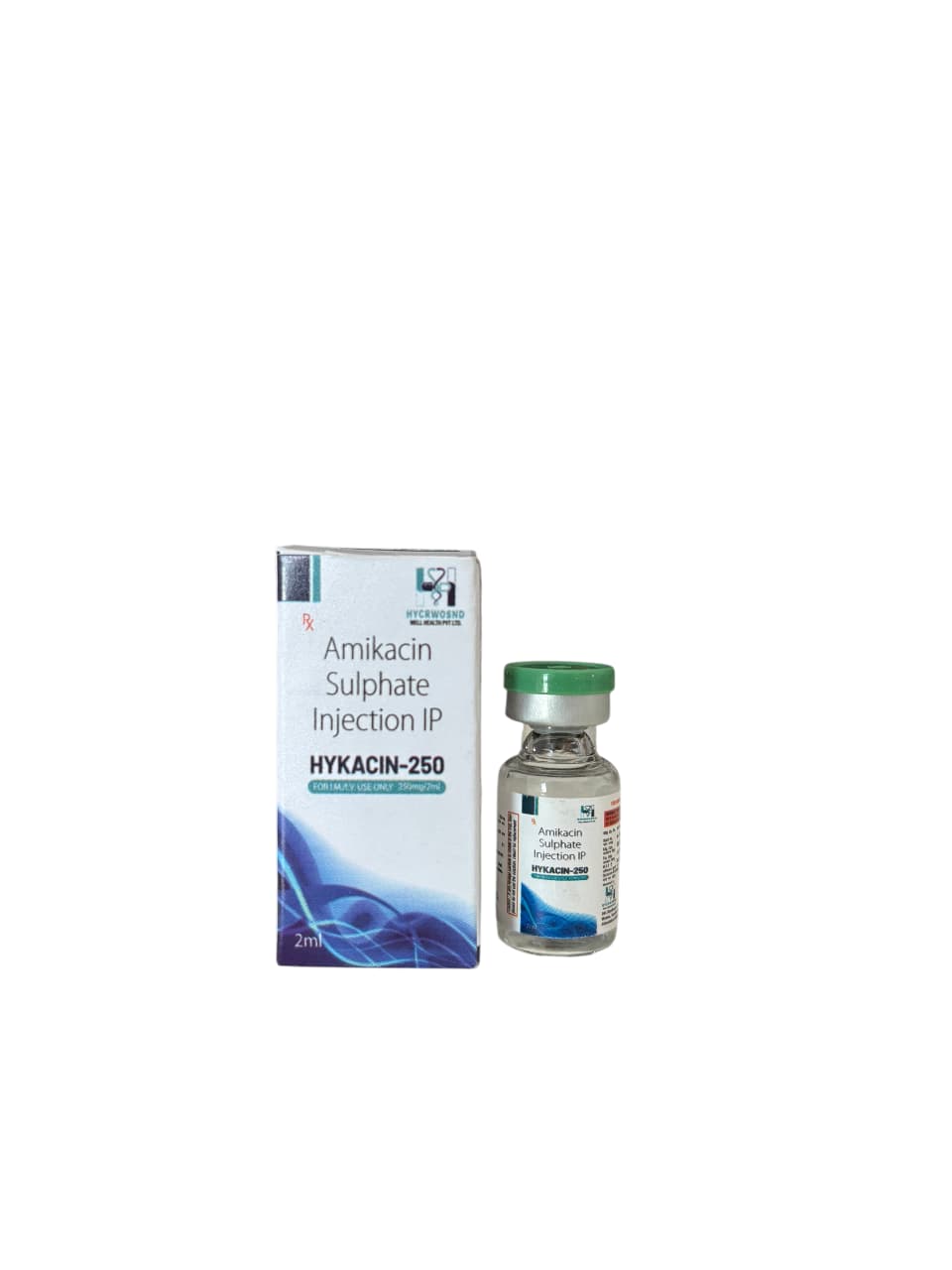
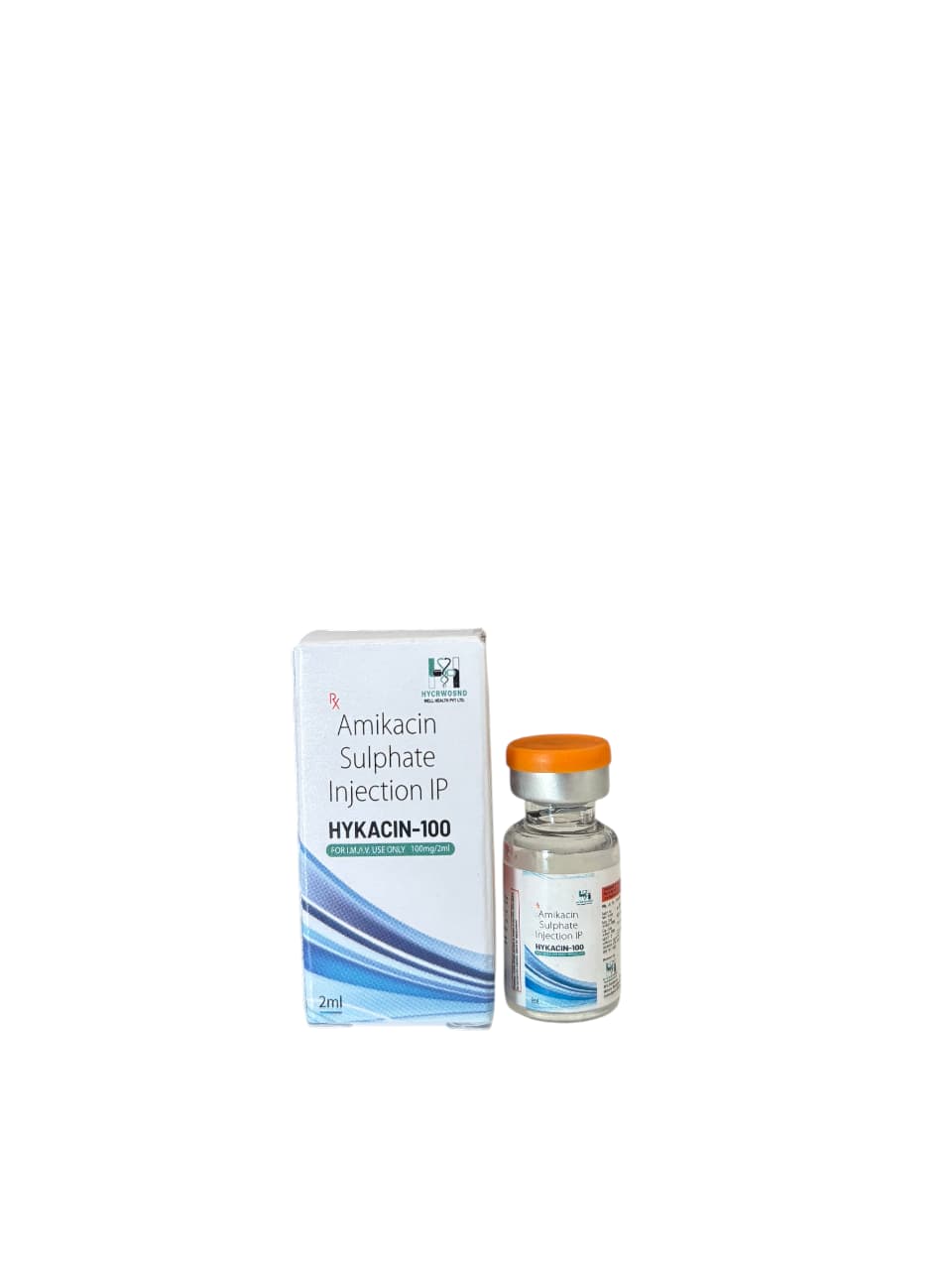
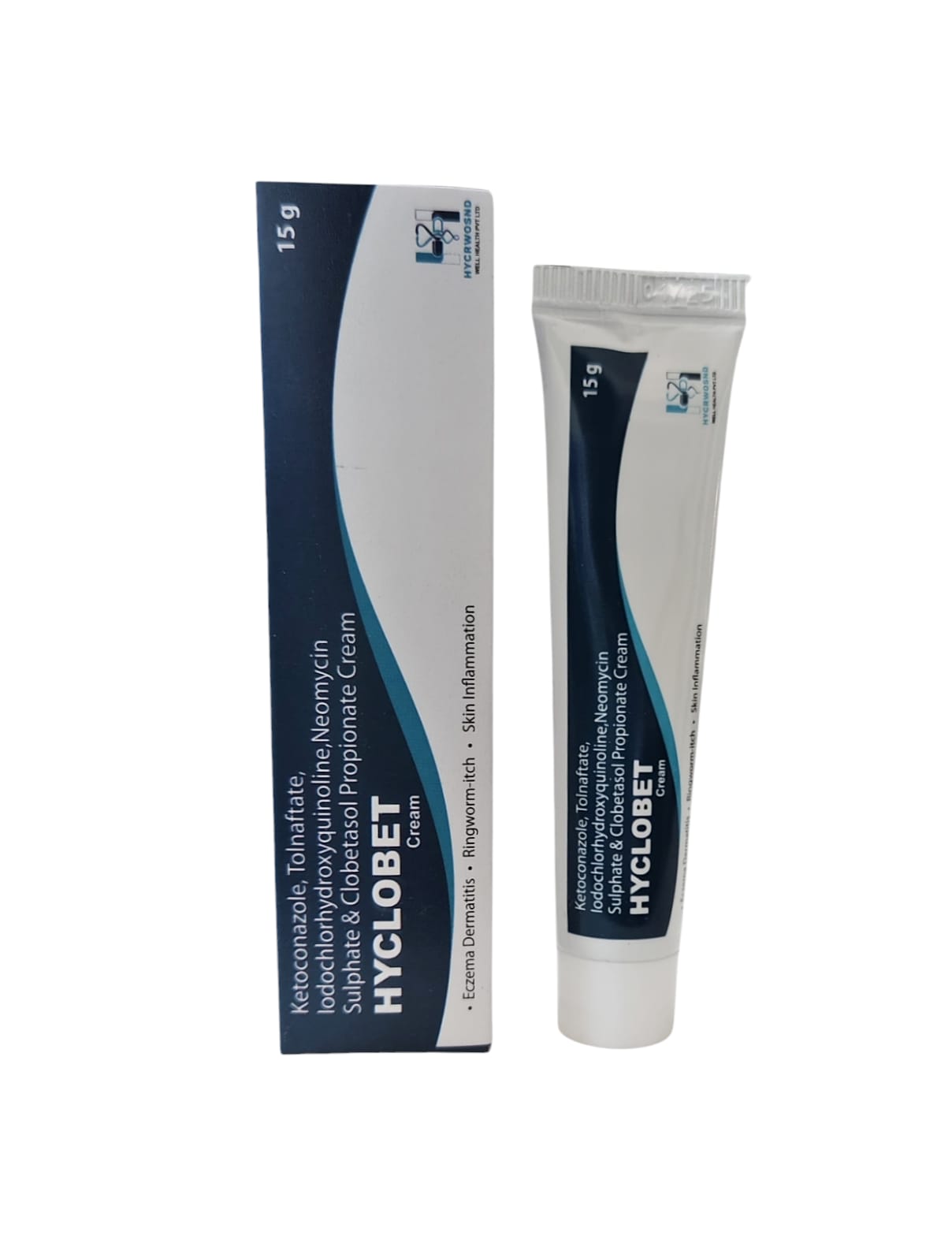
The Start of Our Journey:
In [2023], the journey of HYCRWOSND WELL HEALTH PVT.LTD began with a singular mission—to redefine healthcare through innovation, quality, and unwavering commitment to patient well-being.
The Start of Our Journey:
In [2023], the journey of HYCRWOSND WELL HEALTH PVT.LTD began with a singular mission—to redefine healthcare through innovation, quality, and unwavering commitment to patient well-being.